An important issue in development and use of nanomaterials is their ability to maintain nano-sized dimensions within extended temperature ranges or chemical conditions. Many factors control the stability and physico-chemical properties of TiO2 nanoparticles, including particle size [1], synthesis conditions [2], microstrain and morphology of crystallites [3], doping with metal oxides [4]. In our study the effect of synthesis conditions (water-based or glycol-based) and co-doping with Cr-Sb or V-Sb on the thermal behaviour of titania nanoparticles was investigated by means of temperature resolved synchrotron x-ray diffraction.
Nanocrystalline titania were produced by a novel synthesis route, consisting of a high temperature forced hydrolysis in a coordinating high-boiling solvent (and water for reference). Phase quantification of titania phases (brookite, anatase and rutile polimorphs) was obtained by quantitative Rietveld analysis using GSAS. Volume averaged apparent crystallite sizes were derived by line profile analysis with the Rietveld method (FullProf software). Phase composition and crystallite size are drastically influenced by both synthesis conditions and doping. Synthesis in water resulted in the simultaneous occurrence of anatase and brookite, transformation into rutile begins early but with a slower rate with respect to glycol-based samples (Figure 1A). Doping affected the anatese to rutile tramsformation (A→R), whose onset temperature in undoped titania (715°C) was lowered to 690°C (V-Sb) or prevented up to 950°C (Cr-Sb). Coarsening rate of anatase particle size as a function of temperature follows the A→R sequence. This confirms a particle size control on the transformation process [5].
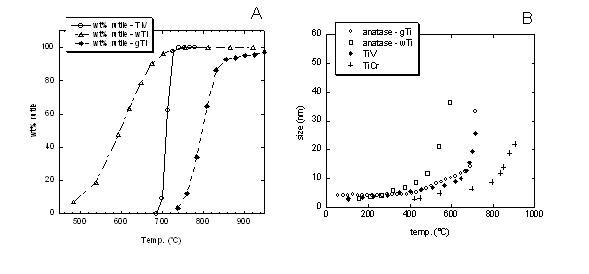 Figure 1. Legend: wTi = undoped titania in water; gTi = undoped titania in glycol; TiV = co-doped V-Sb titania in glycol; TiCr = co-doped Cr-Sb titania in glycol. Figure 1. Legend: wTi = undoped titania in water; gTi = undoped titania in glycol; TiV = co-doped V-Sb titania in glycol; TiCr = co-doped Cr-Sb titania in glycol.
(A) temperature and rate of rutile formation: wTi (470°C) < TiV (690°C) < gTi (715°C) < TiCr (no detected rutile up to 950°C); (B) coarsening rate of anatase particle size as a function of temperature: wTi > gTi ≅ TiV > TiCr
References
[1] Gribb, A. A. & Banfield, J. F. (1997) Particle size effects on transformation kinetics and phase stability in nanocrystalline TiO2. American Mineralogist, 82, 717-728.
[2] Li, Y., White, T. J. & Lim, S. H. (2004) Low-temperature synthesis and microstructural control of titania nano-particles. Journal of Solid State Chemistry, 177, 1372-1381.
[3] Penn, RL; Banfield, JF. (1999) Morphology development and crystal growth in nanocrystalline aggregates under hydrothermal conditions: Insights from titania. Geochimica Et Cosmochimica Acta, 63, 1549-1557.
[4] Reidy, DJ; Holmes, JD; Morris, MA. (2006) The critical size mechanism for the anatase to rutile transformation in TiO2 and doped-TiO2. Journal Of The European Ceramic Society, 26, 1527-1534.
[5] Zhang H., Banfield J. F. (2000) Understanding Polymorphic Phase Transformation Behavior during Growth of Nanocrystalline Aggregates: Insights from TiO2. J. Phys. Chem. B, 104, 3481-3487. |